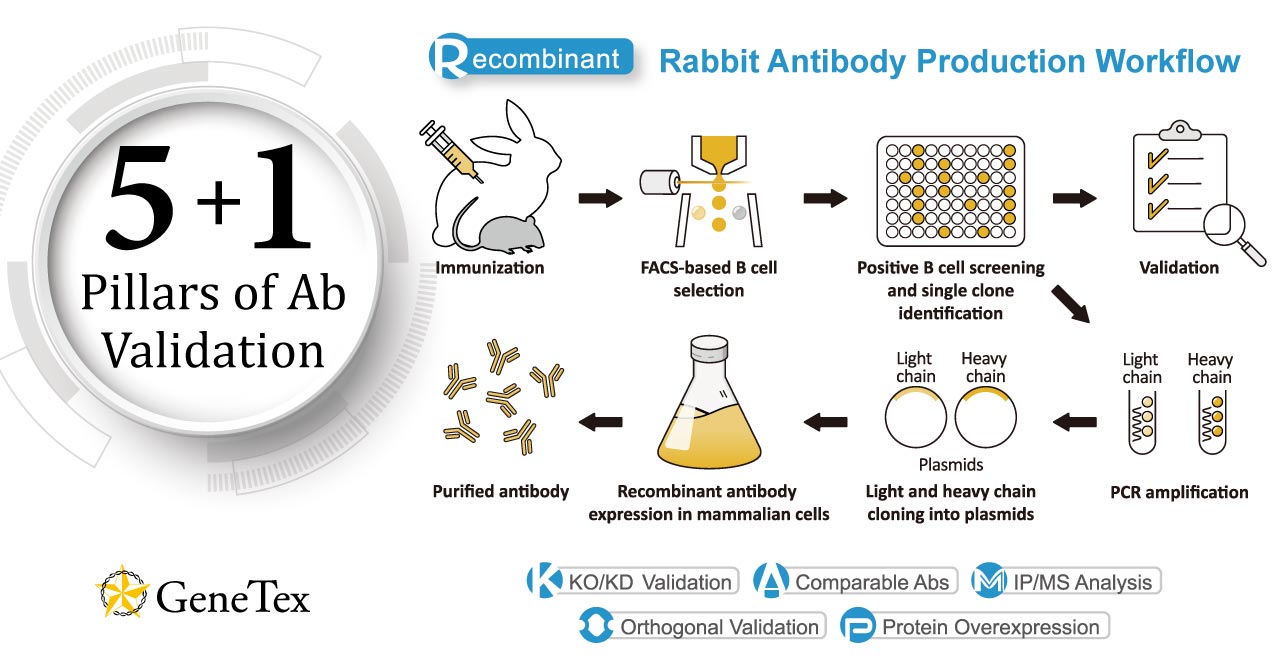
Figure 1. GeneTex’s “5+1” Pillar Plan for recombinant antibody validation.
GeneTex’s production focus is dedicated to the creation of novel recombinant monoclonal antibodies validated using enhanced protocols defined by the International Working Group for Antibody Validation (IWGAV).
Over the last decade, studies published in many of the top scientific journals have clearly described the significant impact of poorly validated antibody reagents on the reproducibility of biomedical research. The unfortunate reality is that the commercial antibody market is compromised by a substantial subset of products that have been demonstrated to be nonspecific. This means that there are two major issues that must be addressed in order to improve the overall quality of the commercial antibody market and therefore the integrity and reproducibility of the biomedical research dependent on antibodies. The first is more widespread adoption of production platforms that emphasize recombinant antibody technology while the second involves incorporation of improved and more coherent validation strategies. GeneTex is making major strides in both of these endeavors.
As was discussed in a prior News feature, GeneTex has already shifted completely to the production of recombinant monoclonal antibodies (Figure 1). Fully recombinant antibodies are defined by their primary sequence, their consistent performance and supply (issues that plague traditional polyclonals and hybridoma-based monoclonals with regard to lot-to-lot variability), and their ability to be engineered (i.e., backbone switching) to meet researcher requirements. The numerous advantages of recombinant antibodies may even evoke more favorable impressions by grant review and journal editorial committees.
In conjunction with the pivot to recombinant antibody production, GeneTex’s second commitment to antibody reliability is its incorporation of enhanced validation protocols, based on the IWGAV proposal mentioned above, into its manufacturing and quality assurance (QA) workflow (1). This involves evaluation of each antibody using at least one, and preferably more, of five validation “pillars” defined in the IWGAV study (1). GeneTex’s version of the IWGAV plan follows the five standard strategies and includes (1) Knockout/Knockdown; (2) Comparable Antibodies; (3) Immunoprecipitation followed by Mass Spectrometry (IP/MS); (4) Biological and Orthogonal Validation; and (5) Recombinant Protein Expression (Figure 1). The company refers to this as its “5+1” Pillar Plan when referencing new recombinant antibody production, with the “+1” designation referring to the pre-validation that is inherent in the application-specific assessment that occurs with clone selection during the recombinant antibody production process (see Validation Examples). With an extensive product catalog, GeneTex is working to replace its traditional polyclonals with new recombinants as well as developing novel antibodies against additional targets. It is also striving to apply IWGAV Five Pillar validation to other products already in the catalog. The task is daunting, but GeneTex is making some progress as noted in a CiteAb blog from October, 2022 (2).
Antibody fidelity, application-dependent reliability, and performance and supply consistency are crucial for ensuring research integrity. GeneTex joins other reputable companies in making antibodies that researchers can trust.
Validation Examples
Knockout / Knockdown  |
| Complete removal or significant reduction of the endogenous signal following genetic strategies involving genomic editing or RNA interference, respectively. |
|
|
Comparable antibodies  |
| Independent antibodies against the same target but to different epitopes, often including distinct samples with different target protein expression levels. |
|
|
|
|
![Influenza A virus Nucleoprotein antibody [HL1089] (GTX636247) Influenza A virus Nucleoprotein antibody [HL1089] (GTX636247)](/upload/media/MarketingMaterial/Article/Five Pillars of Antibody Validation/pic08_255x255.jpg) |
Influenza A virus Nucleoprotein antibody [HL1089] (GTX636247)
|
   |
|
|
IP/MS Analysis  |
| Identification of the target protein immunoprecipitated (IP) by the antibody using mass spectrometry (MS) analysis. |
|
Biological and orthogonal characteristics  |
| Alterations in the endogenous level of the target protein in accordance with specific preparation conditions corresponding to defined biological characteristics, or comparison between antibody-dependent and -independent methods. |
|
|
Protein Overexpression  |
| Tagged target proteins expressed in transfected cells are used as the positive control for validation. |
|
|
References
- Nat Methods. 2016 Oct;13(10):823-7.
- “Supplier antibody validation: how much progress has been made?” (blog.citeab.com/supplier-antibody-validation-progress/)

![Integrin beta 1 / CD29 antibody [HL1255] (GTX636657) Integrin beta 1 / CD29 antibody [HL1255] (GTX636657)](/upload/media/MarketingMaterial/Article/Five Pillars of Antibody Validation/pic01_255x255.jpg)
![NFkB p105 antibody [HL1784] (GTX637436) NFkB p105 antibody [HL1784] (GTX637436)](/upload/media/MarketingMaterial/Article/Five Pillars of Antibody Validation/pic02_255x255.jpg)
![Galectin 3 antibody [HL1878] (GTX637627) Galectin 3 antibody [HL1878] (GTX637627)](/upload/media/MarketingMaterial/Article/Five Pillars of Antibody Validation/pic03_255x255.jpg)
![ZO-1 antibody [HL1133] (GTX636399) ZO-1 antibody [HL1133] (GTX636399)](/upload/media/MarketingMaterial/Article/Five Pillars of Antibody Validation/pic04_255x255.jpg)
![ACE2 antibody [HL1092] (GTX636265) ACE2 antibody [HL1092] (GTX636265)](/upload/media/MarketingMaterial/Article/Five Pillars of Antibody Validation/pic05_255x255.jpg)
![BrdU antibody [HL1111] (GTX636326) BrdU antibody [HL1111] (GTX636326)](/upload/media/MarketingMaterial/Article/Five Pillars of Antibody Validation/pic06_255x255.jpg)
![alpha Synuclein antibody [HL1242] (GTX636641) alpha Synuclein antibody [HL1242] (GTX636641)](/upload/media/MarketingMaterial/Article/Five Pillars of Antibody Validation/pic07_255x255.jpg)
![Influenza A virus Nucleoprotein antibody [HL1089] (GTX636247) Influenza A virus Nucleoprotein antibody [HL1089] (GTX636247)](/upload/media/MarketingMaterial/Article/Five Pillars of Antibody Validation/pic08_255x255.jpg)
![PARP antibody [HL1364] (GTX636804) PARP antibody [HL1364] (GTX636804)](/upload/media/MarketingMaterial/Article/Five Pillars of Antibody Validation/pic09_255x255.jpg)
![RAS (G12D Mutant) antibody [HL10] (GTX635362) RAS (G12D Mutant) antibody [HL10] (GTX635362)](/upload/media/MarketingMaterial/Article/Five Pillars of Antibody Validation/pic10_255x255.jpg)
![Bax antibody [HL236] (GTX635715) Bax antibody [HL236] (GTX635715)](/upload/media/MarketingMaterial/Article/Five Pillars of Antibody Validation/pic11_255x255.jpg)
![GRK2 (phospho Ser670) antibody [HL1035] (GTX635875) GRK2 (phospho Ser670) antibody [HL1035] (GTX635875)](/upload/media/MarketingMaterial/Article/Five Pillars of Antibody Validation/pic12_255x255.jpg)
![Wnt16 antibody [HL1498] (GTX636972) Wnt16 antibody [HL1498] (GTX636972)](/upload/media/MarketingMaterial/Article/Five Pillars of Antibody Validation/pic13_255x255.jpg)
![AKT1 antibody [HL1142] (GTX636413) AKT1 antibody [HL1142] (GTX636413)](/upload/media/MarketingMaterial/Article/Five Pillars of Antibody Validation/pic14_255x255.jpg)
![PD-L1 antibody [HL1041] (GTX635975) PD-L1 antibody [HL1041] (GTX635975)](/upload/media/MarketingMaterial/Article/Five Pillars of Antibody Validation/pic15_255x255.jpg)
![RAI3 antibody [HL1864] (GTX637589) RAI3 antibody [HL1864] (GTX637589)](/upload/media/MarketingMaterial/Article/Five Pillars of Antibody Validation/pic16_255x255.jpg)
![Iba1 antibody [HL22] (GTX635363) Iba1 antibody [HL22] (GTX635363)](/upload/media/MarketingMaterial/Article/Five Pillars of Antibody Validation/pic17_255x255.jpg)
![Vimentin antibody [HL1506] (GTX636980) Vimentin antibody [HL1506] (GTX636980)](/upload/media/MarketingMaterial/Article/Five Pillars of Antibody Validation/pic18_255x255.jpg)
![EpCAM antibody [HL1339] (GTX636759) EpCAM antibody [HL1339] (GTX636759)](/upload/media/MarketingMaterial/Article/Five Pillars of Antibody Validation/pic19_255x255.jpg)
![Gli1 antibody [HL247] (GTX635619) Gli1 antibody [HL247] (GTX635619)](/upload/media/MarketingMaterial/Article/Five Pillars of Antibody Validation/pic20_255x255.jpg)