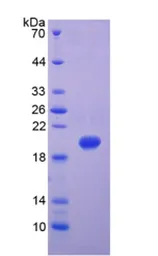
Human MMP9 protein, His tag (Active)
Cat. No. GTX02551-pro
Cat. No. GTX02551-pro
-
ApplicationsFunctional Assay
-
SpeciesHuman