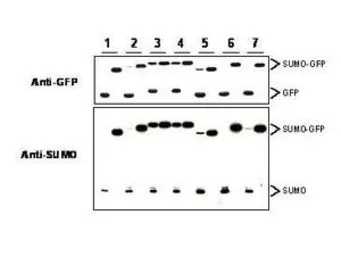
Sumo antibody
Cat. No. GTX48822
Cat. No. GTX48822
-
HostRabbit
-
ClonalityPolyclonal
-
IsotypeIgG
-
ApplicationsWB IP ELISA ChIP assay
-
ReactivityYeast