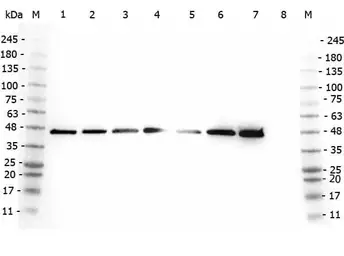
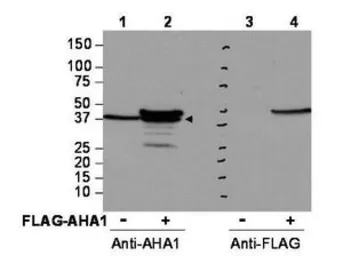
AHA-1 antibody
Cat. No. GTX48723
Cat. No. GTX48723
-
HostRabbit
-
ClonalityPolyclonal
-
IsotypeIgG
-
ApplicationsWB IP ELISA IHC
-
ReactivityHuman, Monkey