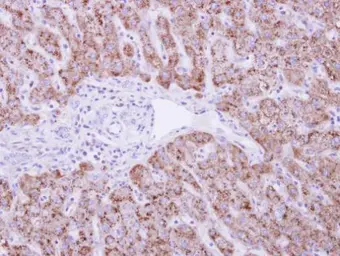
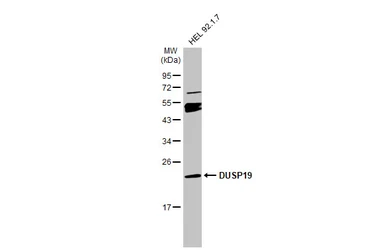
DUSP19 antibody
Cat. No. GTX104197
Cat. No. GTX104197
-
HostRabbit
-
ClonalityPolyclonal
-
IsotypeIgG
-
ApplicationsWB IHC-P
-
ReactivityHuman