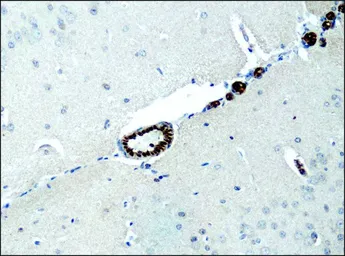
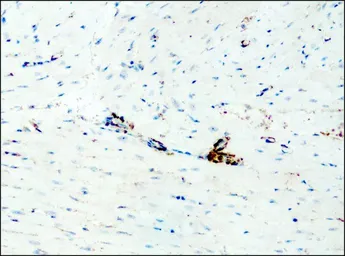
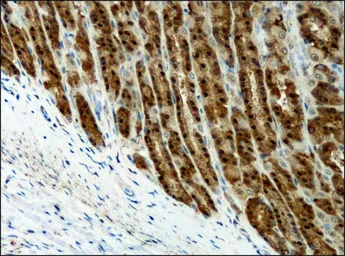
Destrin antibody
Cat. No. GTX11072
Cat. No. GTX11072
-
HostRabbit
-
ClonalityPolyclonal
-
IsotypeIgG
-
ApplicationsWB ICC/IF IHC-P
-
ReactivityHuman, Mouse, Rat, Dog