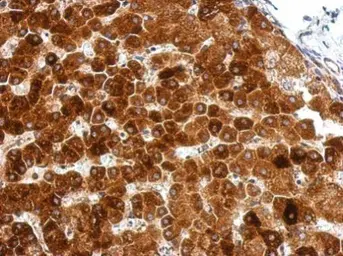
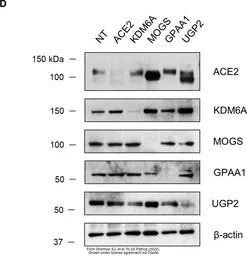
GPAA1 antibody
Cat. No. GTX115131
Cat. No. GTX115131
-
HostRabbit
-
ClonalityPolyclonal
-
IsotypeIgG
-
ApplicationsWB IHC-P
-
ReactivityHuman, Mouse