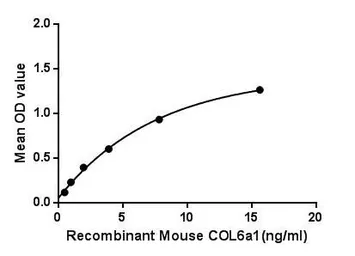
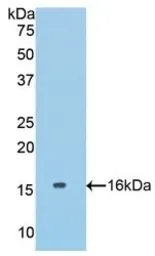
Mouse COL6A1 protein, His tag
Cat. No. GTX00317-pro
Cat. No. GTX00317-pro
-
ApplicationsFunctional Assay
-
SpeciesMouse